Answer:
Efficiency of gasoline engine is 16.6 %
Step-by-step explanation:
It is given that,
A gasoline engine operates at a temperature of, T₁ = 270 C = (270 + 273) K = 543 K
It exhausts at a temperature, T₂ = 180 C = (180 + 273) K = 453 K
The efficiency of this engine is given by :
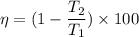
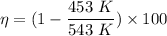
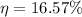
or
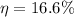
So, the efficiency of this engine is 16.6 %.Hence, the correct option is (d).