Option C
In
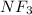 molecule, nitrogen has 5 valence electrons and F has 7 valence electron, 3 electrons of nitrogen are shared with 3 Fluorine atoms and 2 electrons are left on nitrogen atom as lone-pair.
molecule, nitrogen has 5 valence electrons and F has 7 valence electron, 3 electrons of nitrogen are shared with 3 Fluorine atoms and 2 electrons are left on nitrogen atom as lone-pair.
The structure of
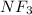 will be pyramidal.
will be pyramidal.
Fluorine is more electronegative than nitrogen, due to this high electronegativity difference the N-F bond is polar in nature. There is net dipole moment in
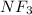 molecule thus, it is asymmetric in nature.
molecule thus, it is asymmetric in nature.
The structure of
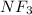 is in the attached file.
is in the attached file.
Therefore, it has polar bonds and it is asymmetric.