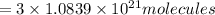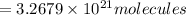Answer:
Molecules of aspirin we are consuming is
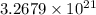 .
.
Step-by-step explanation:
Mass of an aspirin in 1 alka-seltzer tablet = 324 mg = 0.324 g
Molar mass of an aspirin,
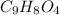 = 180 g/mol
= 180 g/mol
Moles of an aspirin in one tablet =
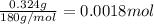
Number of molecules in 0.0018 moles of an aspirin:
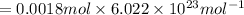
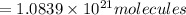
Number of molecules in of aspirin in 1 tablet:
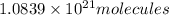
Number of molecules in of aspirin in 3 tablet: