The question is incomplete, here is the complete question:
Sodium carbonate
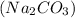 is formed from the reaction between
is formed from the reaction between
A: a weak acid and a strong base
B: a strong acid and a strong base
C: a strong acid and a weak base
D: a weak acid and a weak base
Answer: The given salt is formed from the reaction between a weak acid and strong base.
Step-by-step explanation:
Salts are formed when an acid reacts with a base during a neutralization reaction.
- When a strong acid and a weak base reacts, it leads to the formation of acidic salt.
- When a strong base and weak acid reacts, it leads to the formation of basic salt.
- When a strong acid and strong base or weak acid and weak base reacts, it leads to the formation of neutral salts.
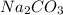 is a salt which is formed by the combination of
is a salt which is formed by the combination of
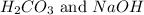 . Carbonic acid is a weak acid and sodium hydroxide is a strong base. So, the given salt is a basic salt.
. Carbonic acid is a weak acid and sodium hydroxide is a strong base. So, the given salt is a basic salt.
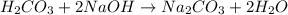
Hence, the given salt is formed from the reaction between a weak acid and strong base.