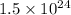The surface area is given
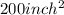 and thickness is 0.005 inch thus, volume will be:
and thickness is 0.005 inch thus, volume will be:
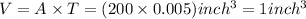
Converting
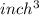 to
to
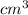
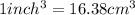
The density of Nickel is
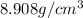 thus, mass will be:
thus, mass will be:
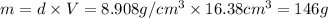
Now, molar mass of nickel is 58.7 g/mol thus, number of moles can be calculated as follows:
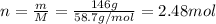
in 1 mole there are
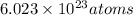 thus, number of atoms in 2.48 mol will be:
thus, number of atoms in 2.48 mol will be:
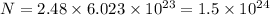
Therefore, number of atoms of nickel required is