The density of a material is its unique property which is different for different substances. The volume or mass of a compound can be determined from the other given mass of a compound, if the density of both the material is available. We know density of a material is equal to the mass per unit volume. From the given data 13.6 g of mercury has 1 cm³ volume. Thus 28.5g of mercury will have
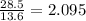 cm³ volume. Now as per the given density value of lead 11.4g of lead has 1 cm³ volume. Thus 2.095 cm³ volume of lead has (2.095×11.4)=23.889g.
cm³ volume. Now as per the given density value of lead 11.4g of lead has 1 cm³ volume. Thus 2.095 cm³ volume of lead has (2.095×11.4)=23.889g.