Answer:-
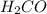 has higher vapor pressure.
has higher vapor pressure.
Explanations:- Weaker the attraction forces, higher is the vapor pressure. Both the given compounds that is methanol and formaldehyde are polar and so they have dipole-dipole forces of attraction. Apart from this, methanol has hydrogen bonding also as hydrogen is bonded to more electron negative oxygen atom. This hydrogen bonding makes it to have stronger attraction forces and so the vapor pressure is lower. Formaldehyde doesn't have hydrogen bonding and so the forces are comparatively weaker that makes it to have higher vapor pressure.