(a) The diameter of cylindrical water reservoir is
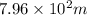 and its height is 45.54 m.
and its height is 45.54 m.
Radius of cylinder is half of its diameter thus,
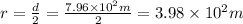
Volume of cylinder can be calculated as follows:
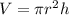
Here, r is radius and h is height, putting the values,
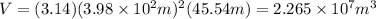
Since,
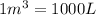
Thus, volume will be
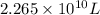
The concentration of fluoride
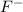 is approximately 1 ppm.
is approximately 1 ppm.
Since, 1 ppm=1 mg/L
thus,
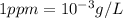
Mass can be calculated as follows:
m=C×V=
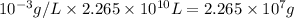
Therefore, mass of fluoride will be
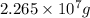 .
.
(b) In NaF, sodium and florine are present in 1:1 ratio thus, 1 mole of fluoride gives 1 mole of NaF.
Mass of fluoride is
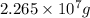 and its molar mass is 19 g/mol thus, number of moles will be:
and its molar mass is 19 g/mol thus, number of moles will be:
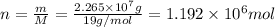
Thus, number of moles of NAF formed will be
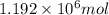
Molar mass of NaF is 41.98 g/mol, mass can be calculated as follows:
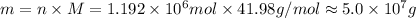 .
.
Therefore, grams of NaF containing this much of fluoride is
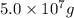 .
.