Answer:
23.33 grams of element b was produced by second sample of the compound on decomposition.
Step-by-step explanation:
Sample-1 of compound containing ab gives :
Mass of a = 15 g
Mass of b = 35 g
Ratio of mass of mass of a to mass of b:
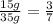
Sample-2 of compound containing ab gives :
Mass of a = 10 g
Mass of b = x
Ratio of mass of mass of a to mass of b:
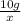
Since the both samples are of same compound the ratio of masses will remain same.
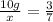
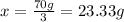
23.33 grams of element b was produced by second sample of the compound on decomposition.