The overall reaction is given by:
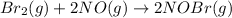
The fast step reaction is given as:
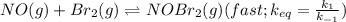
The slow step reaction is given as:
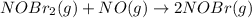 (slow step
(slow step
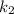 )
)
Now, the expression for the rate of reaction of fast reaction is:
![r_(1)=k_(1)[NO][Br_(2)]-k_(-1)[NOBr_(2)]](https://img.qammunity.org/2019/formulas/chemistry/high-school/jcbn2ddupp5dq5fgjxe32lc6ethfl3ijjh.png)
The expression for the rate of reaction of slow reaction is:
![r_(2)=k_(2)[NOBr_(2)] [NO]](https://img.qammunity.org/2019/formulas/chemistry/high-school/k9jjn86w7yxiddjjsy632gahfidl45lvjy.png)
Slow step is the rate determining step. Thus, the overall rate of formation is the rate of formation of slow reaction as
![[NOBr_(2)]](https://img.qammunity.org/2019/formulas/chemistry/high-school/5d9jmmo65rs5scf6ho3cfs7kzaz56geok1.png) takes place in this reaction.
takes place in this reaction.
The expression of rate of formation is:
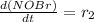
=
![k_(2)[NOBr_(2)][NO]](https://img.qammunity.org/2019/formulas/chemistry/high-school/kutvtiubvodtrb2ljtrxo7s2fgjc6clycf.png) (1)
(1)
Now, consider that the fast step is always is in equilibrium. Therefore,
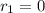
![k_(1)[NO][Br_(2)]= k_(-1)[NOBr_(2)]](https://img.qammunity.org/2019/formulas/chemistry/high-school/amxjcv27dxbethb17arn3rb3vbfjykjh8k.png)
![[NOBr_(2)] = (k_(1))/(k_(-1))[NO][Br_(2)]](https://img.qammunity.org/2019/formulas/chemistry/high-school/yuxkttibsumrewki12ui91s41r7p70aljl.png)
Substitute the value of
![[NOBr_(2)]](https://img.qammunity.org/2019/formulas/chemistry/high-school/5d9jmmo65rs5scf6ho3cfs7kzaz56geok1.png) in equation (1), we get:
in equation (1), we get:
![(d(NOBr))/(dt)=k_(2)[NOBr_(2)][NO]](https://img.qammunity.org/2019/formulas/chemistry/high-school/orxlitusipa8s1vftuzq05po02o8jg4z7m.png)
=
![k_(2) (k_(1))/(k_(-1))[NO][Br_(2)][NO]](https://img.qammunity.org/2019/formulas/chemistry/high-school/ruginf1kxuyi8npr2b0lgliw4sp7lw9dzu.png)
=
![(k_(1)k_(2))/(k_(-1))[NO]^(2)[Br_(2)]](https://img.qammunity.org/2019/formulas/chemistry/high-school/4m5g8qp1r9h0u090xo1qk3qgxft3i6rapy.png)
Thus, rate law of formation of
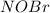 in terms of reactants is given by
in terms of reactants is given by
![(k_(1)k_(2))/(k_(-1))[NO]^(2)[Br_(2)]](https://img.qammunity.org/2019/formulas/chemistry/high-school/4m5g8qp1r9h0u090xo1qk3qgxft3i6rapy.png) .
.