Answer:-
3.77 x 10²¹ arsenic(iii) oxide molecules correspond to an LD50 value of 0.0146 for a 187 lb man
Explanation: -
Weight of man = 187 lb
=
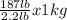
= 84.8 Kg
LD50 = number of grams of a substance that is lethal to 50% of animals per kilogram of body weight
0.0146 =
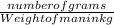
Thus
Number of grams of lethal substance = 0.0146 x 84.8
= 1.24 g
Molar mass of arsenic(iii) oxide = 197.841 g/mol
Number of moles of arsenic(iii) oxide =
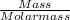
=
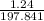
= 0.00627 moles
Number of molecules of arsenic(iii) oxide = Number of moles x Avogadro number
= 0.00627 x 6.02 x 10²³
= 3.77 x 10²¹