Answer:-
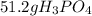 .
.
Solution:- The given balanced equation is:
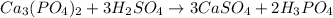
From this equation, there is 1:2 mol ratio between calcium phosphate and phosphiric acid. The mol ratio between sulfuric acid and phosphoric acid is 3:2.
First of all we convert the grams of each reactant into moles and then the moles of each are multiplied by the above mol ratios to get the moles of phosphoric acid. Moles of phosphoric acid are multiplied by it's molar mass to get it's grams. The reactant that gives less grams of phosphoric acid would be the limiting reactant and this amount of phosphoric acid formed will be our answer.
The calculations for grams of phosphoric acid calculated from each of the reactants are as follows:
Calculations of phosphoric acid grams from given grams of calcium phosphate:
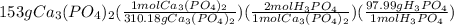
=
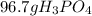
Calculations for grams of phosphoric acid from given grams of sulfuric acid:
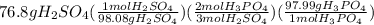
=
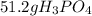
From above calculations, sulfuric acid gives least amount of phosphoric acid. It means sulfuric acid is limiting reactant and the amount of phosphoric acid formed is 51.2 g.