Answer:- selenium, Se.
Explanations:- When an element gain electrons then it becomes negatively charged means it forms it's anion. Total electrons of the anion are the sum of electrons and the charge of the anion. In our problem, the element is q and it forms
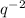 ion. The Total electrons for the anion
ion. The Total electrons for the anion
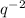 = electrons of q + 2
= electrons of q + 2
As per the given info, there are 36 electrons in
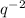 ion.
ion.
So, 36 = electrons of q + 2
electrons of q = 36 - 2
electrons of q = 34
q, the neutral atom has 34 electrons means it's atomic number is 34. If we check out the periodic table then the element with atomic number 34 is Selenium, Se.