First identify the molecular formula of ammonium sulphide to determine the number of hydrogen atoms present the molecule.
ammonium sulphide =
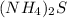
According to molecular formula, there are eight hydrogen atoms present in the molecule.
Thus, moles of hydrogen atom is calculated by the multiplication of number of hydrogen atoms and moles of ammonium sulphide.
Number of moles of hydrogen atom =
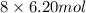
= 49.6 moles
Now, 1 mole of atom = Avogadro number (
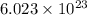 )
)
Number of hydrogen atoms =
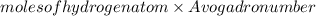
=
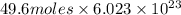
=
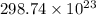
=
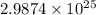
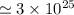 atoms of hydrogen
atoms of hydrogen
Hence,
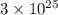 is the number of hydrogen atoms.
is the number of hydrogen atoms.