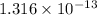The fraction of gas phase molecules is calculated by the division of final pressure to the initial pressure.
Fraction =
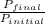 (1)
(1)
Here, initial pressure = 1.0 atm
final pressure =
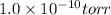
First, convert torr into atm
1 atm = 760 torr
final pressure =
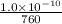
=
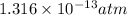
Now, put the value of initial and final pressure in formula (1)
Fraction =
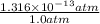
=
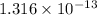
Thus, fraction of the gas phase molecules =