The given concentration of boric acid = 0.0500 M
Required volume of the solution = 2 L
Molarity is the moles of solute present per liter solution. So 0.0500 M boric acid has 0.0500 mol boric acid present in 1 L solution.
Calculating the moles of 0.0500 M boric acid present in 2 L solution:
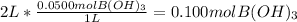
Converting moles of boric acid to mass:
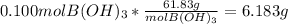
Therefore, 6.183 g boric acid when dissolved and made up to 2 L with distilled water gives 0.0500 M solution.