Diameter of a single hydrogen atom = 212 pm
Calculating the length of a row of
 Hydrogen atoms:
Hydrogen atoms:
 Hydrogen atoms *
Hydrogen atoms *
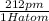
=
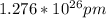
Converting the length from pm to km:
The conversion factor for converting pm to m is
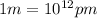
The conversion factor from m to km is
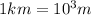
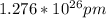 *
*
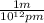 *
*
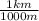 =
=
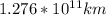
Therefore,
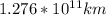 is the distance in kilometers when
is the distance in kilometers when
 Hydrogen atoms are placed in a row.
Hydrogen atoms are placed in a row.