The Henderson-Hasselbalch equation is:
![pH = pK_a + log ([conjugate base, (A^(-))])/([weak acid, (HA)])](https://img.qammunity.org/2019/formulas/chemistry/college/ar5h9t94y6hf7wcgok9a7y0bfnong4vwxw.png)
From the above equation.,
- When concentration of both the forms that is the concentration of conjugate base and weak acid are equal then pH =
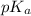 .
.
![[conjugate base, (A^(-))] = {[weak acid, (HA)]}](https://img.qammunity.org/2019/formulas/chemistry/college/b45yu5o6oiotpisbty4cr238426iui8g19.png)
![pH = pK_a + log ([weak acid, (HA)])/([weak acid, (HA)])](https://img.qammunity.org/2019/formulas/chemistry/college/olu3t4sdqds0fcds3wo311ko9vz4r5mpjf.png)
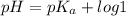
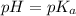
- When pH <
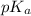 , protonated species.
, protonated species. - When pH >
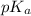 , deprotonated species.
, deprotonated species.
pH of
 is 10 and
is 10 and
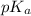 is 4.8. (given).
is 4.8. (given).
Since, pH >
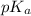 so, deprotonated form of
so, deprotonated form of
 will be predominant that is
will be predominant that is
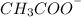 .
.
The structure of the predominant form of
 is shown in the image.
is shown in the image.