Answer:-
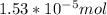 .
.
Solution:- Aspartame is a artificial sweetener with molecular formula
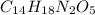 and it's molecular mass is 294.3 gram per mol.
and it's molecular mass is 294.3 gram per mol.
We have been given with 4.50 mg of aspartame and asked to calculate the moles. For mass to moles conversion we divide the given mass in grams by molar mass.
Since, the mass is given in mg, we need to convert it to g first.
1 mg = 0.001 g
So,
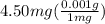
= 0.00450 g
Now let's calculate the moles on dividing the grams by molar mass as:
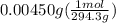
=
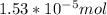
Hence,
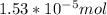 are present in 4.50 mg of aspartame.
are present in 4.50 mg of aspartame.