Answer:
See detailed explanation.
Step-by-step explanation:
Hello!
In this case, for the described chemical reaction, we can proceed as follows:
A) For the complete chemical reaction we note down every reacted and produced species as well as the proper balancing process:
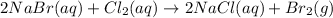
In which gaseous bromine may give off.
B) The dissociated ionic equation requires the ionization of the aqueous species in ions, expect for chlorine which is not ionized:
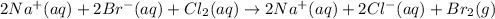
C) For the net ionic equation we cancel out the sodium ions as they are at both reactants and products:
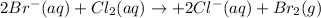
D) Based on C) we infer that the spectator ions here are the sodium ions.
Best regards!