Answer : 108 kJ of heat is absorbed
Explanation :
Enthalpy of vaporization of water at body temperature is 2.41 kJ/g
That means we need 2.41 kJ to evaporate 1 gram of sweat.
Let us use this as a conversion factor to find the amount of heat needed to evaporate 45 grams of sweat.
The equation can be set as follows.
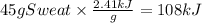
Therefore we can say that 108 kJ of heat is absorbed when sweat is evaporated from someone's skin.