Answer : The pH of the solution is, 11.8
Solution : Given,
Concentration (c) = 0.1 M
Base dissociation constant =
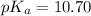
Now we have to calculate the value of
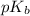 .
.
As we know that,
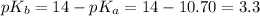
Now we have to calculate the value of
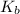 .
.
The expression used for the calculation of
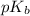 is,
is,
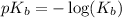
Now put the value of
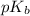 in this expression, we get:
in this expression, we get:
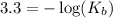
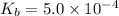
The given equilibrium reaction is,
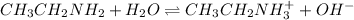
initially conc. 0.1 0 0
At eqm. (0.1-x) x x
Formula used :
![k_b=([CH_3CH_2NH_3^+][OH^-])/([CH_3CH_2NH_2])](https://img.qammunity.org/2019/formulas/chemistry/college/y03gtolh9y18p9477csgdrvu61o9wzycua.png)
Now put all the given values in this formula ,we get:
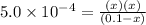
By solving the terms, we get:
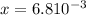
Now we have to calculate the concentration of hydroxide ion.
![[OH^-]=x=6.8* 10^(-3)M](https://img.qammunity.org/2019/formulas/chemistry/college/rk1a6ukpd6mwevq41go8dfzf5codhimpxq.png)
Now we have to calculate the pOH.
![pOH=-\log [OH^-]](https://img.qammunity.org/2019/formulas/chemistry/high-school/e2d5i1z7zr97bzmljv86uipm68wd8z6hty.png)
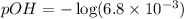
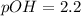
Now we have to calculate the pH.
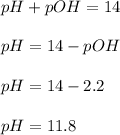
Therefore, the pH of the solution is, 11.8