Answer:
The value of z is 10,
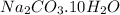 .
.
Step-by-step explanation:
Mass of washing soda = 2.123 g
Mass of sodium carbonate = 0.787 g
Mass of water = 2.123 g-0.787 g =1.336 g
Wasing soda → Sodium carbonate + water
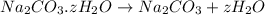
Moles of sodium carbonate =
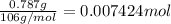
Moles of water =
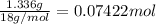
According to reaction , 1 mol of sodium carbonate obtained with z moles of water.
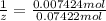
z = 9.99 ≈ 10