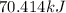The process in which slid phase is converted into gaseous phase directly without achieving the liquid phase is said to sublimation.
The heat required to completely sublime is calculated by the product of number of moles and heat of sublimation.
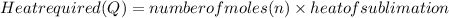 (1)
(1)
And, heat of sublimation of carbon dioxide is 32.3 kJ/mol
Number of moles of carbon dioxide =
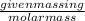
=
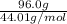
=
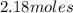
Now, put the values in formula (1)
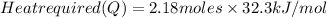
=
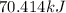
Hence, heat required to completely sublime is