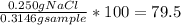The balanced chemical equation representing the reaction of NaCl with AgNO3 is,

The balanced chemical equation representing the reaction of KBr with AgNO3 is,

Moles of
 =
=
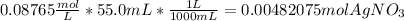
0.00482075 mol
 completely removes Chlorides and bromides in the sample.
completely removes Chlorides and bromides in the sample.
Mole ratio of
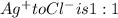
Mole ratio of
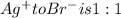
So, the total moles of chloride and bromide in the sample = 0.00482075 mol
Let mass of NaCl be x g
Mass of KBr be y g
Total mass of sample = 0.3146 g
=> x + y = 0.3146 g
x = 0.3146 -y
Total number of moles of NaCl + KBr = 0.00482075 mol
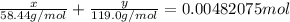
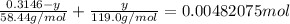
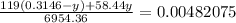
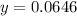
Therefore mass of KBr = 0.0646 g
Mass of NaCl = 0.3146 - 0.0646 g = 0.250 g
Mass % of NaCl in the sample =