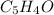Given the mass of CO2 =15.02 g
Moles of
 =
=
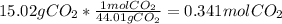
Mass of H2O = 2.458 g
Moles of
 =
=
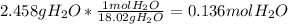
Moles of C =
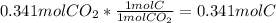
Moles of H =
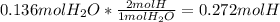
Mass of C in the sample =
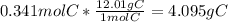
Mass of H =
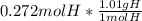 =0.275 gH
=0.275 gH
Mass of O in the sample = 5.467 g - (4.095 g +0.275 g) = 1.097 g O
Moles of O =
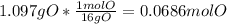
Simplest mole ratios of the elements in the compound:
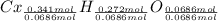
Therefore the empirical formula of the compound is