2d(g) + 3e(g) + f(g) -------> 2g(g) + h(g)
As it can be seen from the equation that for every 3 moles of e consumed,=2 moles of g formed.
for every 1 moles of e consumed =
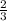 moles of g formed
moles of g formed
So, if e is decreasing at a rate of 0.16 mol s⁻¹
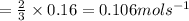
So when e is decreasing at 0.16 mol s⁻¹, g is increasing at a rate of 0.106 mol s⁻¹.