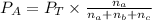Partial pressure can be calculated from balanced equation as :
Partial pressure can be calculated from total pressure and mole fraction of a molecule. The equation of partial pressure is given as:
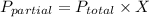
Mole fraction can be calculated from number of moles of different substances, that we can get from a balanced equation.
If a balanced equation is as follows:
aA+bB---->cP
Mole fraction of A will be =
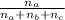
And partial pressure of A will be: