Number of moles is defined as the ratio of given mass in grams to the molar mass of compound.
Number of moles =
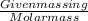
Now, put the value of given mass of
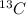 in grams and molar mass of
in grams and molar mass of
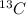 in g/mol i.e. 13 g/mol.
in g/mol i.e. 13 g/mol.
Thus,
number of moles =
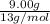
= 0.692 mol
Hence, number of moles of
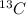 = 0.692 mol
= 0.692 mol