The average atomic mass of copper = 63.546 amu
Copper has two natural isotopes.
The % abundance of the first isotope Cu-63 is 69.17 %
Mass of the isotope Cu-63 = 62.940 amu
The percentage abundance of other isotope will be 100 - 69.17 % = 30.83 %
Let the atomic mass of other isotope be x amu
Average atomic mass is the weighted average of the atomic masses of all isotopes of an element.
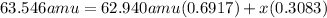
x = 64.90 amu
The atomic mass of other isotope of copper is 64.90 amu
Therefore, the isotope will be Cu-65