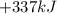The change in enthalpy refers to the amount of heat absorbed or heat evolved in a reaction which is carried out at constant pressure.
The change in enthalpy of a reaction is equal to the sum of formation of products minus sum of formation of reactants. It is denoted by
 .
.
The given reaction is:
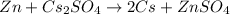
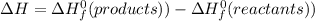
Substitute the given values
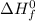 (products) and
(products) and
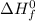 (reactants) in above formula, we get
(reactants) in above formula, we get
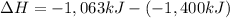
=
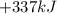
Thus, change in enthalpy for the single replacement reaction is