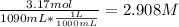Given the molality of CaCl2 solution = 3.17 m
That means 3.17 mol
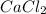 are present per 1 kg water.
are present per 1 kg water.
Mass of
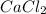 =
=
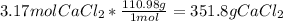
Mass of solution = Mass of solvent + Mass of Solute = 1000 g + 351.8 g = 1351.8 g solution.
Density of solution = 1.24 g/mL
Volume of the solution =
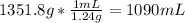
Molarity is the moles of solute per L of the solution
Calculating molarity of the solution: