Every element is represented by a nuclear symbol, which contains of three parts: (a) the atomic number, (b) the symbol and (c) the mass number of the specific isotope of the element. The symbol is represented as:
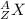
where, X is the chemical symbol of the element, A is mass number (sum of number of protons and neutrons in the nucleus), and Z is atomic number (number of protons in nucleus).
The atomic symbol of silicon is
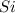
Atomic number of isotope of silicon,
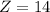
Mass number of silicon,
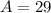
So, the nuclear symbol for the isotope of silicon is
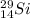 .
.