Alright, lets get started.
If you have a density of 3g/ml and a volume of 10 ml
means density = 3 g / ml
Volume = 10 ml
Formula for density, mass, volume will be
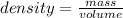
Plugging the value
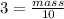
Multiplying 10 in both sides
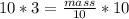
mass = 30 gm
Hence the answer is 30 gm. : Answer
Hope it will help :)