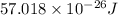Answer:
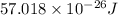
Energy is directly proportional to the frequency.
The energy of a photon is given by:
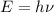
Where, E is the energy and
 is the frequency.
is the frequency.
Frequency of the radio wave is given:
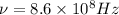

Multiply the above two:
Energy,
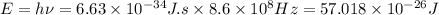
Hence, the energy of each photon of radio wave having frequency
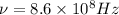 is
is