The two different isotopes have weights :
w1 = 78.918 amu
w2 = 80.916 amu
average weight w3 = 79.903 amu
The mixing of two components can be modeled as
let the fraction of w1 be 'x'
hence
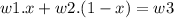
now this is a linear equation in 'x'. Substituting the values we get
x = 0.507
hence the percentage of Br79 = 50.7% and the percentage of BR81 = 49.3%