Mass of solute, W_B = 8.05 g[/tex]
Molar mass of solute, M_B = 32 g /mol
Mass of solvent,
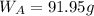
Molar mass of solvent,
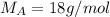
Number of mol of solvent is
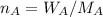
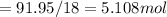
Number of mol of solute is
n_B = W_B /M_B
= 8.05 / 32 = 0.25 mol
Mole fraction of solvent is the number of moe of solvent divided by total nmber of mole of solute and solvent as follows:
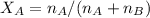
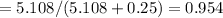
Thus, mole fraction of solvent is 0.954