Converting the temperature, 295 K from Kelvin to Celsius scale:
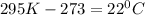
Water has a boiling point of
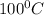 and a melting point of
and a melting point of
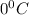
When we compare water at two different temperatures,
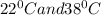 we can say that water is in liquid form at both these temperatures as both of them are quite below the boiling temperature and above the melting temperature.
we can say that water is in liquid form at both these temperatures as both of them are quite below the boiling temperature and above the melting temperature.
The temperature difference between water at the given two temperatures =
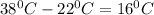
Water at
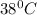 is at a higher temperature and so is warmer than water at a lower temperature of
is at a higher temperature and so is warmer than water at a lower temperature of
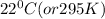 .
.