Answer:
8 molecules of oxygen gas are required.
Step-by-step explanation:
Combustion reaction is the type of chemical reaction in which a hydrocarbon reacts with oxygen gas to give to carbon dioxide and water.
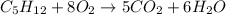
According to reaction ,1 mole of pentane reacts with 8 molecules of oxygen gas to give 5 moles of carbon dioxide and 6 moles of water.
So, to burn 1 mole of pentane we will need 8 molecules of oxygen gas.