Given, the density of water is 0.9975 g/ml. Density of water is mass of water per unit volume. Mass of 1 ml of water supposed to be 0.9975 g from density of water. So, mass of 10 ml of water is (0.9975 X 10) g= 9.975 g. From graduated cylinder, mass of 10 ml water is measured to be 9.955 g. So, error for mass of 10 ml water= (9.975-9.955)=0.02 g. Percentage of error for 10 ml water is
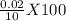 = 0.2. Error in the mass for the 10 ml of water is 0.2 %.
= 0.2. Error in the mass for the 10 ml of water is 0.2 %.