Aspirin is acetylsalicylic acid with chemical formula C₉H₈O₄. This is an anti-pyretic drug used to treat pain, fever, or inflammation. Aspirin contains polar functional group i.e, carboxylic acid group (-COOH) which can form hydrogen bonds with polar water molecules and gets dissolved.
450 ml water can dissolve 1g of aspirin, so, 1.5 ml water can dissolve
 = 3.33 x
= 3.33 x
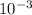
So, 3.33 X 10⁻³ g of aspirin would be lost in the 1.5 ml of water.