Initially, weigh the correct amount of
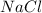 calculated from the formula.
calculated from the formula.
Number of moles =
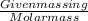
As 1 mole of
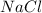 is given and molar mass of
is given and molar mass of
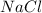 is
is
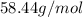 , then
, then
1 mole of
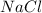 =
=
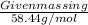
Mass of
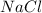 in g =
in g =
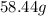
Thus,
1 mole of
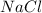 = 58.44 g.
= 58.44 g.
Now, the weighed amount of
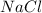 i.e. 58.44 g is added to a 1 liter container and then add small amount of water in the container to dissolve the salt. After that, fill the container with distilled water to the graduation mark or until the total volume reaches 1 liter.
i.e. 58.44 g is added to a 1 liter container and then add small amount of water in the container to dissolve the salt. After that, fill the container with distilled water to the graduation mark or until the total volume reaches 1 liter.
Thus, option (c) is correct i.e. first put one mole of salt in the container then add the water with stirring till the total volume reaches 1 liter.