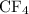
Step-by-step explanation
The IUPAC names binary covalent compounds similar to ionic compounds. The first part of the systematic name "carbon" indicates the component with a partial positive charge- carbon
 in this case. The second part indicates the name of the component with a partial negative charge as if it was an anion. "Fluoride" stands for fluorine
in this case. The second part indicates the name of the component with a partial negative charge as if it was an anion. "Fluoride" stands for fluorine
 whereas the prefix "tetra-" indicates that there are four fluorine atoms per molecule. The first species in the IUPAC name- carbon in this case- carries no prefix meaning that only a single carbon atom is present in each carbon tetrafluoride molecule.
whereas the prefix "tetra-" indicates that there are four fluorine atoms per molecule. The first species in the IUPAC name- carbon in this case- carries no prefix meaning that only a single carbon atom is present in each carbon tetrafluoride molecule.
Atoms with a partial positive charge shall be placed in front of those with a partial negative charge. Hence write
 and then
and then
 ; indicate the number of the atom present in each molecule with a subscript. There are four fluorine atoms in each carbon tetrafluoride molecule- thus write a "4" as the subscript. There shall be no subscript attached to carbon since each carbon tetrafluoride molecule contains only one carbon atom.
; indicate the number of the atom present in each molecule with a subscript. There are four fluorine atoms in each carbon tetrafluoride molecule- thus write a "4" as the subscript. There shall be no subscript attached to carbon since each carbon tetrafluoride molecule contains only one carbon atom.