Answer : The final temperature of Aluminium is 17° C
Explanation :
The rubbing alcohol evaporates at 25 °C . That means it changes from liquid state to gaseous state. This process is known as Vaporization and the amount of heat it absorbs is known as enthalpy of vaporization.
Step 1 : Find moles of alcohol
We have 1.5 g of C₃H₈O.
Let us find the moles of alcohol.
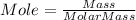
Molar mass of rubbing alcohol is 60.1 g/mol
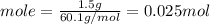
Step 2 : Find heat absorbed by given amount of alcohol
The enthalpy of vaporization of rubbing alcohol is 45 kJ/mol
The amount of heat absorbed by alcohol can be calculated as follows.
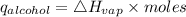
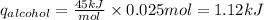
So during the process of evaporation, alcohol absorbs 1.12 kJ of heat.
This heat is taken from the aluminium.
Step 3 : Equate the q values for alcohol and aluminium
Therefore we have ,
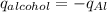
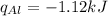
Step 4 : Find delta T for Al using the heat value found in the above step
Heat lost by Aluminium can be calculated using following equation
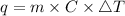
Here q is the amount of heat lost by Al. We need to convert q from kJ to J
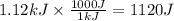
C is the specific heat of Al which is 0.9 J/gC
ΔT is the change in temperature of Aluminium.
m is the mass of Al which is 150 g.
Let us plug in the values and solve for ΔT
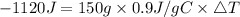
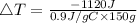
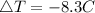
Step 5 : Find final temperature using delta T
But ΔT is the difference in final temperature and initial temperature.
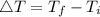

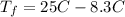
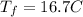
The final temperature of Aluminium is 17° C