Lead chloride rises five fold in solubility on heating at temperature of
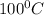 .
.
Solubility is defined as the capability of solute to dissolve in the solvent. It can be determine in terms of maximum quantity of particle or solute is dissolved in a solvent at equilibrium.
Five fold in solubility refers to the five times increase in solubility. Maximum five folds of solubility can be obtained from lead chloride
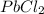 .
.
At room temperature, lead chloride is not soluble in water but in hot water lead chloride is soluble in water i.e. at
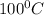 .
.
Thus, five folds in solubility can be obtained from lead chloride in comparison to other chlorides as they don't give five folds in solubility.