The attractive force between two isolated ions is given by the formula:
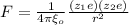 -(1)
-(1)
where,
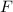 is force of attraction,
is force of attraction,
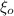 is permittivity of vacuum =
is permittivity of vacuum =
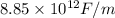 ,
,
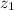 and
and
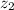 are valence of two ions,
are valence of two ions,
 is the electron charge =
is the electron charge =
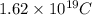 and
and
 is the inter-atomic distance.
is the inter-atomic distance.
The inter-atomic distance,
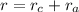
where,
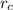 is the radius of cation and
is the radius of cation and
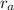 is the radius of anion.
is the radius of anion.
Since value of radius of cation is given so,
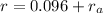 -(2)
-(2)
The cation and anion are divalent so the value of
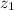 and
and
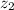 is 2 and -2 of cation and anion respectively.
is 2 and -2 of cation and anion respectively.
Substituting the values in formula (1):
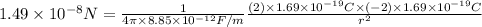
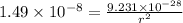
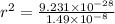
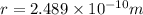 =
=
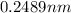
Substituting this value of
 in equation 2:
in equation 2:
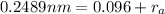
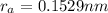
Hence, the radius of anion is
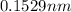 .
.