The indication from which one can determine how much of active ingredient is recovered after purification method is said to be a percent recovery.
The formula for percent recovery is:
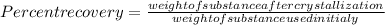
The solubility of acetanilide in 100 ml of chloroform at
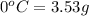
The solubility of acetanilide in 100 ml of chloroform at
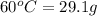
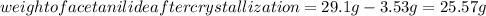
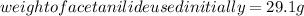
Substituting the values in the above formula:
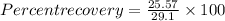
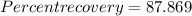 %.
%.
Hence, the maximum percent recovery that can be achieved for the recrystallization of acetanilide from chloroform is
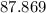 %.
%.