Answer : The correct option is, (C) There is more AB than A and B at equilibrium, and the reaction favors the products.
Explanation :
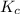 is defined as the equilibrium constant. It is defined as the ratio of concentration of products to the concentration of reactants.
is defined as the equilibrium constant. It is defined as the ratio of concentration of products to the concentration of reactants.
Or, it is determined by multiplying the concentrations of products together and divided by the concentrations of the reactants.
The equilibrium reaction is :
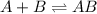
The expression for equilibrium constant will be,
![K_c=([AB])/([A][B])](https://img.qammunity.org/2019/formulas/chemistry/middle-school/8d6jgjpga7qywvv9v8yt9wf6bkiebeyl14.png)
where,
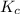 = equilibrium concentration
= equilibrium concentration
[AB] = concentration of AB = 72 % = 0.72 M
[A] = concentration of A = 14 % = 0.14 M
[B] = concentration of B= 14 % = 0.14 M
![K_c=([0.72])/([0.14][0.14])=36.7](https://img.qammunity.org/2019/formulas/chemistry/middle-school/cdzfbp3jcsb9l2kwix9nlppgngf6zfgsty.png)
There are 3 conditions which are :
When
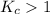 ; then the reaction is product favored.
; then the reaction is product favored.
When
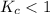 ; then the reaction is reactant favored.
; then the reaction is reactant favored.
When
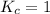 ; then the reaction is in equilibrium.
; then the reaction is in equilibrium.
As per question, the value of
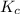 is greater than 1. So, the reaction is product favored.
is greater than 1. So, the reaction is product favored.
Hence, the correct option is, (C) There is more AB than A and B at equilibrium, and the reaction favors the products.