The general formula for alkane and cycloalkane are
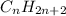 and
and
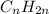 , respectively.
, respectively.
Axial and equatorial hydrogen atoms are inter-converted during a ring flip .
The anti conformation is lower in energy than a gauche conformation.
The increase in energy when tetrahedral bond angles deviate from the optimum angle of 109.5° is known as angle strain.
Now,
Alkanes are saturated hydrocarbon with general formula
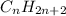 and cycloalkanes are also saturated cyclic hydrocarbons where carbons are linked in the form of a ring with general formula
and cycloalkanes are also saturated cyclic hydrocarbons where carbons are linked in the form of a ring with general formula
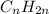 .
.
The phenomenon which involves the inter-conversion of axial hydrogen atoms into equatorial hydrogen atoms is said to be ring flip.
The energy of the anti conformation is lower than the gauche conformation due to steric strain, which is lower in anti conformation and results in low energy.
The increase in potential energy of the molecule because of the deviation of bond angles from its original values is said to be angle strain.