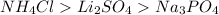By using the formula of depression in freezing, it can easily identify the rank of the given compounds.
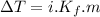
where,
 = freezing point depression
= freezing point depression
i = van 't Hoff factor (number of ions per individual molecule of solute)
m = molality of the solute
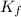 = molal freezing point
= molal freezing point
According to question, if concentration (m) and
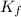 is same, then only van 't Hoff factor will change.
is same, then only van 't Hoff factor will change.
For
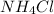 , i = 2
, i = 2
For
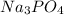 , i = 4
, i = 4
For
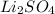 , i= 3
, i= 3
Now, the solute with the largest i value results in low freezing point.
The highest value of i is 4 (
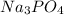 ), thus
), thus
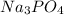 has lowest freezing point.
has lowest freezing point.
And,
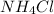 has highest freezing point.
has highest freezing point.
The order of freezing point is :